The extinct bird could be back as early as 2028, followed by other species that have vanished
The year 1681 was the last time mankind saw the dodo before it became the universal symbol for extinction. The flightless bird, endemic to Mauritius, was said to be so trusting that it would walk right up to Dutch sailors―who found it on the island in the early 1600s―and offer them its nest. As it had no natural predators, it had no reason to doubt the humans. It was wrong. Within decades, hunting, deforestation and damage caused by animals that the humans introduced to the island wiped out the dodo.
Now, centuries later, the same humans are trying to create a new version of the dodo, one that is not “dumb”. And, it won’t be alone. Scientists are also working on bringing back the woolly mammoth and the thylacine, a Tasmanian marsupial last seen in the early 19th century. Colossal Biosciences, a Texas-based “de-extinction company” that uses genetic engineering to “revive” extinct species, says the dodo will be with us in 2028. Its team of scientists has been assembling and sequencing the dodo genome (the total amount of DNA in an organism) using DNA extracted from a skull in the collection of the Natural History Museum of Denmark. Children of the 1990s will remember how scientists used mosquito DNA to bring back dinosaurs in Michael Crichton’s Jurassic Park. This, though, seems more realistic.
“Our mission is to make extinction a thing of the past,” Beth Shapiro, an evolutionary biologist and chief science officer at Colossal, tells THE WEEK. “About half of the species on earth are threatened with extinction in the next 50 to 100 years, and much of this is due to changes in habitat because of humans. We should be improving and increasing the number of tools and options available to us in terms of how we might protect species, genetic diversity and ecosystems.”
At a time when a UN report ‘Nature’s Dangerous Decline Unprecedented’ warns of an “accelerating” pace of species extinction, and the World Animal Foundation predicts that up to half of all species could be extinct by 2050, this attempt at “de-extinction” offers hope. And Colossal has already raised $200 million to aid its dodo, mammoth and thylacine project. The choice of the three animals―a bird, a regular mammal and a marsupial―was intentional. With these three, Colossal will cover a lot of ground in terms of animal types for future projects.
As per a World Economic Forum report, half of the global GDP is dependent on nature to some extent, and biodiversity loss and ecosystem collapse could bring global GDP down significantly. “In many cases, there are ecosystems where the absence of a keystone animal or plant that used to be there is causing instability that is potentially leading other species to extinction,” says Shapiro. “And if we can use this growing diversity of tools to stop that from happening, then we would be able to stop so many species from becoming extinct.”
 A representational image of a woolly mammoth | Colossal
A representational image of a woolly mammoth | Colossal
Collecting old DNA is at the heart of Colossal’s work. “When we go and collect a bone from the Arctic, I can take a little piece of that bone, grind it up and extract DNA,” says Shapiro. “The DNA that I get could have been damaged―microbes get in there and chew up the DNA, there is shearing of the DNA strands [and] damage accumulates. With the help of AI and computational advances, scientists are now able to get that DNA out of those bones and then piece them together like a giant puzzle.”
It all begins with looking for the closest living relative to the animal one is trying to bring back. In the case of the mammoth, that’s the Asian elephant―they share 98 per cent of the same DNA. “So when the scientists have an Asian elephant cell growing in a dish in a lab, they already have 98 per cent of a mammoth,” Shapiro explains in a YouTube video. “We just have to engineer the remaining 2 per cent. [To do that,] we go out and collect mammoth genomes (there are frozen mummies in the Arctic), which we’ve done already. We can line up the [mammoth and Asian elephant genomes] on a computer next to each other and identify where they are different from each other and yet where all of those mammoth genomes are the same as each other. These are the portions of the mammoth genome that are important to make a mammoth look and act like a mammoth. Then we use the tools of genome editing (like CRISPR) to gradually tweak those Asian elephant cells in a dish to be more mammoth-like. We now have our mammoth-ised Asian elephant cells in a dish. We use somatic cell nuclear transfer, commonly known as cloning, to transform those cells into an embryo, and we implant that embryo into a surrogate maternal host (an Asian elephant). The embryo develops, builds into a beautiful little mammoth-ised Asian elephant, and it is born, and it looks and acts like a mammoth and it lives happily ever after.”
The characteristics that the Asian elephant cell takes on would include fuzzy hair and a layer of blubber unique to the woolly mammoth, says Shapiro. “We hope to be able to have mammoths by 2028; right now the teams are on track to have embryos by late 2026,” she says.
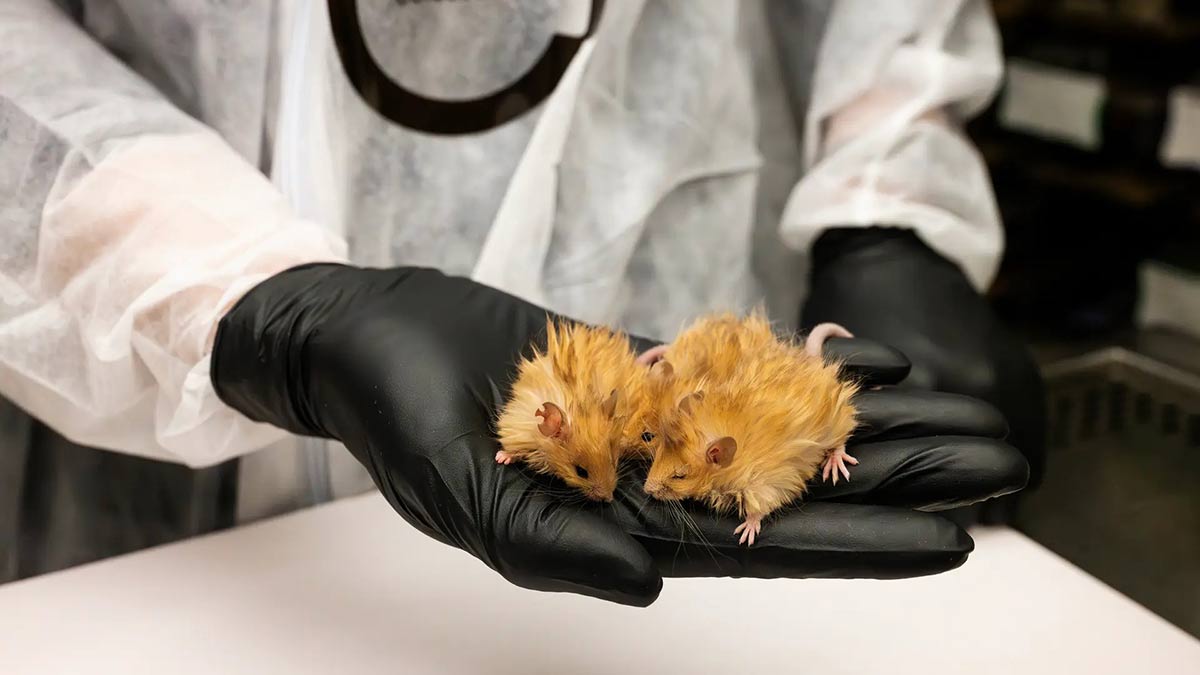 In the right direction: Woolly mice created by Colossal | Colossal
In the right direction: Woolly mice created by Colossal | Colossal
As for the dodo, the Nicobar Pigeon is the closest living relative, and the Solitaire, a type of extinct bird that was found on the island of Rodrigues, east of Madagascar, is its closest genetic relative. For surrogacy, the company plans to use chickens, which are abundant and research-friendly.
Importantly, the de-extinction process for birds is different from mammals. In the mammoth, for instance, once the mammoth-ised Asian elephant cell is ready, its nucleus is taken out and put in place of the regular nucleus in a fertilised Asian elephant egg, says Colossal’s website. The egg then begins to divide and grow into an embryo. According to the Natural History Museum in London, “While a cell’s nucleus, which contains an organism’s DNA, is easy to locate in mammals, it is much harder to locate in a developing bird egg simply due to its size (the egg is a single cell). Even if the nucleus is found, scientists are yet to find a way to remove, edit and then reinsert it back into the egg after it has begun to form.”
And so, Colossal plans to take primordial germ cells―which are precursors to reproductive cells―from a Nicobar pigeon embryo grown in a lab. They would then genetically modify these cells to make them dodo-like. These modified cells would then be inserted into the embryos of a sterile chicken and rooster. The chicken and rooster will hatch from their eggs and grow up with reproductive cells that have dodo-like traits. And then, hopefully, their offspring would resemble a dodo.
Every species that is a candidate for de-extinction has a different set of challenges. “The surrogate for the thylacine is a dunnart, which is a tiny marsupial mouse, while the thylacine is a marsupial carnivore, the size of a wolf,” says Shapiro. “So, the challenge there is how do we change that little mouse into this beautiful, iconic, striped, large-jawed marsupial wolf.”
There is also the challenge of time. “[For the mammoth,] we have to put these embryos in an elephant, and wait for the gestation period of 22 months,” says Shapiro. Given that Asian elephants aren’t highly distributed in the wild, access to their sperm and eggs is extremely limited.
So, the Colossal team developed induced pluripotent stem cells (iPSCs) from Asian elephant tissue―these are stem cells that can transform into any type of cell in the body. With this, theoretically, Colossal can edit the iPSCs into sperm and egg cells of an elephant instead of getting it from the actual elephant.
As for the long gestation period, Colossal looked at a mouse, which has a gestation period of only 20 days. The results would be quicker, and if they did end up giving mammoth-like traits to a mouse, it would be a sort of validation of their efforts. “There is a 200-million-year evolutionary distance between a mammoth and a mouse, and we wouldn’t want to put a mammoth gene in a mouse and see what happens because it will be hard to interpret and we don’t know if it’s going to be safe for the mouse,” says Shapiro. “Instead, we narrow down genes in a mammoth to those that are associated with the code phenotype (observable physical characteristics)―woolly-ness, colour, length, texture, etc., and then we look at the mouse literature. There are so many lab mice that have been sequenced and tested in different ways. So here’s a gene that’s identified as potentially important for mammoths; here’s the same gene but the mouse version of it.”
Colossal scientists modified ten of the mice genes, some of them related to fur texture and colour, based on their study of mammoth genes. They used an advanced genome editing technique called multiplex editing, which allows scientists to edit several genes at once. The company put out the results on March 4, in a series of images and videos of a cute woolly mouse. “We have been able to test it in a mouse model, which means that we can do it in a very safe and ethical and fast way,” says Shapiro. “So it’s a great validation of our approach and also a model for testing these hypothesis.”
However, there are people in the scientific community who do not fully believe in Colossal’s plans and point out that most of their work is yet to be peer-reviewed. Also, there are several risks of reintroducing animals into the wild, something Shapiro acknowledges. “Well, as of now the goal is to restore interactions between species and preserve biodiversity,” she says. “Science is paving the way to resurrect the past. If we are to make room for extinct species in the real world, we as a society will have to alter our attitudes, our actions and even our laws.”
But how far can they go? Could they bring back the Neanderthals? “Well, we have a little bit of Neanderthal DNA in our own genomes, somewhere between 2 and 4 per cent, depending on one’s ancestry. If we were to go around the world and just collect the parts of Neanderthal DNA that exists in people who are alive today, we could put together about 92 per cent of the Neanderthal genome. Which makes you ask if they are really extinct. However, we couldn’t get ethical consent for a Neanderthal to be brought back and so I will personally draw a line at that.”